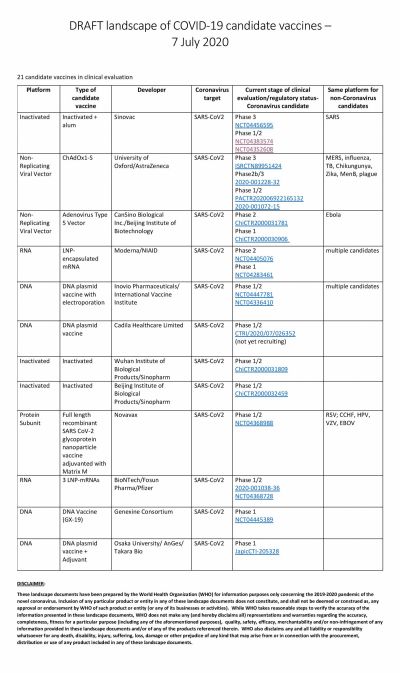
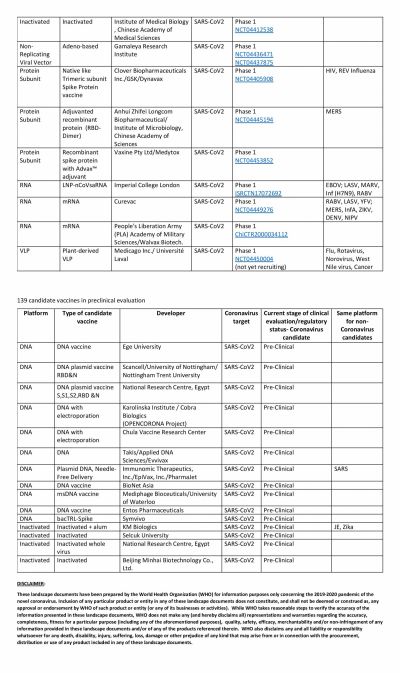
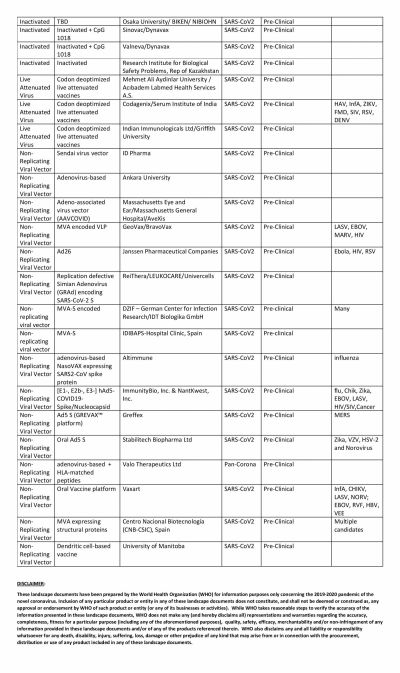
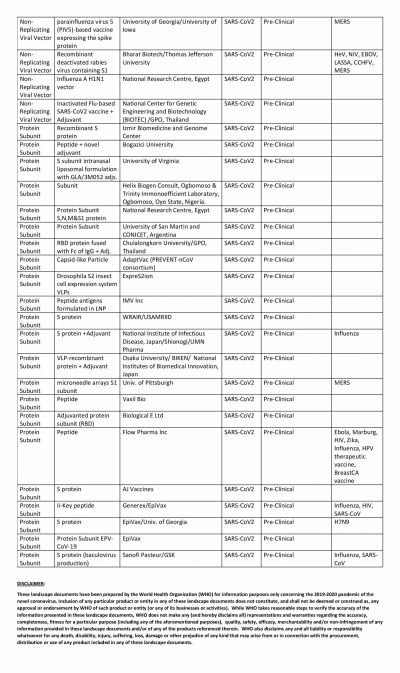
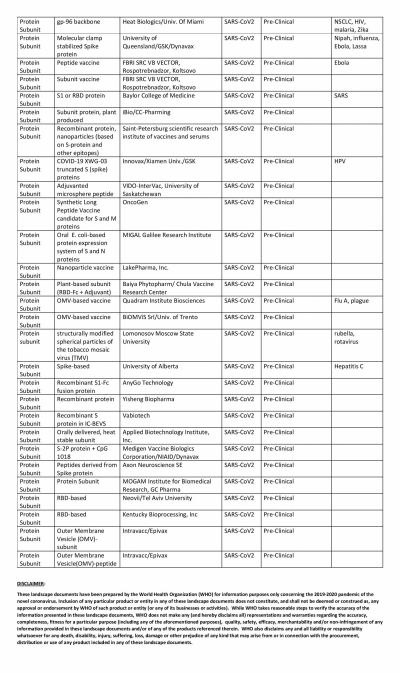
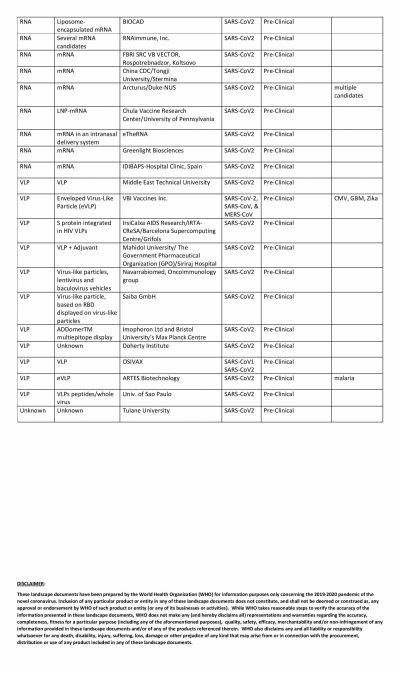
The global COVID-19 vaccine race is in full swing with several researchers claiming that their vaccines show promise. Even though the United States could have been the one to beat with numerous biotech and pharmaceutical companies present in the country, it has been looking at its oldest ally the U.K. to produce a vaccine at the earliest.
America's own vaccine, which is being tested by Moderna, has somewhat fallen off the grid and is trailing in the race. The RNA-based vaccine has not been able to enter Phase III of clinical trials. Novavax, the potential vaccine candidate based on protein subunit, is also in Phase II while Inovio is in Phase I.
Why Is Moderna Trailing?
Moderna offered hopes to millions of Americans wanting to get back to their normal life when they began Phase II trials in May. However, since then, the company backed by the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), has not made any significant strides.
Instead, Moderna, which has never produced a vaccine before, has fallen out with US scientists for failing to deliver timely trial protocols and its resistance to taking experts' advice on how to run the study. Sources told Reuters that if Moderna was a little cooperative, the delays would have been avoided and close to half a billion dollars that were funded by the government would not have been in jeopardy.

Nevertheless, the Massachusetts-based company has denied the allegations and claimed that there were differences of opinion. "It has not been smooth or easy. No one has ever done anything like this before — not Moderna, not the NIH, and not any of the other companies," said Ray Jordan, Moderna spokesperson.
AstraZeneca-Oxford Vaccine
As Moderna lagged, the vaccine jointly developed by the University of Oxford and AstraZeneca in the UK took leaps. Currently, under Phase III trials, the ChAdOx1 nCoV-19 vaccine is being tested in England, Brazil, and South Africa. The developers say that they can deliver emergency vaccines by October this year.
"The clinical studies are progressing very well and we are now initiating studies to evaluate how well the vaccine induces immune responses in older adults and to test whether it can provide protection in the wider population," said Professor Andrew Pollard, head of the Oxford Vaccine Group, in a statement earlier this month (July).
Vaccines Developed in China
However, in this regard, China seems to be leading the race with multiple Chinese biotech companies running at least eight trials simultaneously. The most promising of them is the one from CanSino Biologics. After showing positive results in Phase I trial, the vaccine moved to Phase II where the Ad5-nCoV vaccine was able to produce a strong immune response. That led the Chinese Military to approve the vaccine in limited numbers.
In May, Canada allowed human trials with the Ad5-nCoV vaccine and Ian Stewart, the president of the National Research Council of Canada said that the "vaccine candidate holds great promise".
Sinopharm and Sinovac Vaccine Candidates
Two other vaccines to enter Phase III are also from China. The vaccine from Sinopharm, a state-owned pharmaceutical company, is based on the inactivated virus and has successfully conducted Phase I and II trials among 1,120 volunteers. According to the company, among volunteers who received two doses of the vaccine, 100 percent antibody production was observed in 28 days. Overall, the vaccine candidate showed a 97.6 percent antibody-positive conversion rate. Phase II trials are ongoing in the United Arab Emirates (UAE).
The other company in Phase III trials is Sinovac. The private company's vaccine, named CoronaVac entered Phase II trials in June and involved 743 volunteers while the Phase III trial began in Brazil earlier this month. In the South American country, the trial is supported by Instituto Butantan.
Russia's Answer to COVID-19 Vaccine
Russia's answer to the other COVID-19 vaccines is the one developed by Gamaleya Research Institute. The research organization claims to have completed clinical trials. Skeptics would suggest it is an impossible feat to achieve as the Russian institute's timeline suggests that they have completed Phase II and III trials in less than a month.
However, according to Sechenov University Center for Clinical Research on Medications, which collaborated with Gamaleya in the trial said it was successful and the volunteers will be discharged on July 15 and 20. However, the number of trial volunteers is not encouraging. In Phase I, which began on June 18, an intramuscular solution of the vaccine was tested at the Burdenko Military Hospital on 18 participants, while Phase II involved a mere 20 volunteers who were given doses on June 23.
"The research has been completed and it proved that the vaccine is safe," said Sechenov University Center for Clinical Research on Medications head and chief researcher Elena Smolyarchuk. Considering that and also other vaccine candidates that are in Phase II, will Moderna come out on top? Only the time can tell.