
One of the most promising and hopefully the best medicine against the Coronavirus infection, Remdesivir has failed to prevent deaths among COVID-19 patients, revealed a WHO-sponsored a study that included over 11,000 people from 30 countries.
The data was posted online Thursday, October 15 but it is to be peer-reviewed or published in a scientific journal. As per Dr. Ilan Schwartz, an infectious-disease physician at the University of Alberta in Canada, this recently revealed data "puts the issue to rest" as there is no certainty that Remdesivir can decrease the mortality rate among the Coronavirus patients.
But Dr. Peter Chin-Hong, an infectious-disease expert at the University of California, San Francisco said that a huge trial similar to this one was conducted in several countries with different healthcare systems and it can lead to inconsistent treatment protocols whose effects can be quite different to analyze.
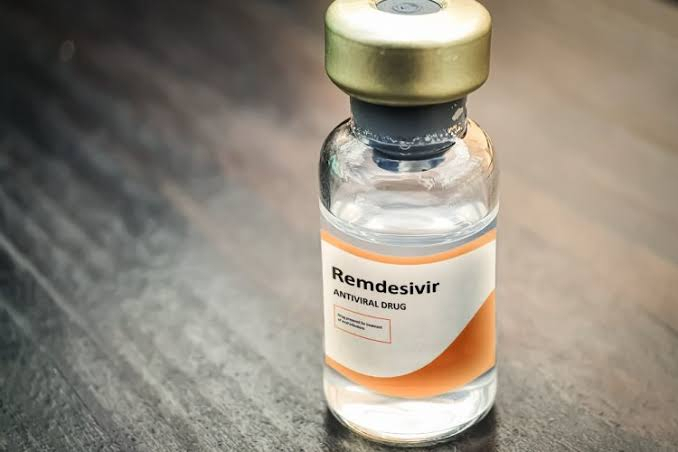
A Failed Drug?
This medicine was first developed to treat Ebola and hepatitis C and in May this year, the US Food and Drug Administration gave the emergency authorization to use Remdesivir to treat Coronavirus patients.
This happened after a trial by the National Institutes of Health (NIH) found that the anti-viral drug modestly reduces the recovery time of severe COVID-19 patients. But the study did not claim that this medication can prevent deaths in patients.
However, the drug has become a part of the standard of care for Coronavirus-infected patients in the US. It has been administrated to thousands of COVID-19 patients since the FDA approved it, and even the US President Donald Trump was given this antiviral medication after he tested positive for COVID-19 early this month.
Even though Remdesivir received the actual clearance only for its usage in critical cases, the emergency authorization was expanded in August to include all the hospitalized Coronavirus patients—despite the COVID-19 severity. But many criticized this move and said that the federal agency had made this shift without any sufficient evidence.
'Solidarity Trial'
The huge worldwide trial supported by WHO—Solidarity trial—enrolled over 11,300 Coronavirus-infected adults in 405 hospitals, located in 30 different countries. The trial participants have been receiving four drugs separately, including Remdesivir, or in combination with other drugs -- hydroxychloroquine, lopinavir, interferon, or interferon plus lopinavir. In this trial, around 4,000 people did not receive any of these medications.
But as it was found, none of these medicines reduced the mortality rate. There are some previous studies that pointed out the futility of hydroxychloroquine and lopinavir as a Coronavirus treatment, while for interferon, less data has been published as of now.
However, healthcare experts noted that the antiviral, Remdesivir might be still helpful and benefit people suffering from the SARS-CoV-2 caused disease early in the course of their illness. They believe that administrating the drug when a COVID-19 patient's immune system is completely destroyed by the virus, may be pointless.









