A tiny prick from a mosquito can have deadly consequences when the lethal Malaria-causing parasite, Plasmodium, enters the human body. A new study, however, finds that pathogen's infectious ways are more nefarious than previously known. According to the paper, the parasite triggers genes in human cells which manipulates the body's adaptive immune responses and prevents it from attacking the protozoan.
In the study, researchers from NYU Abu Dhabi (NYUAD) described the discovery of a new by immune evasion strategy employed by the Plasmodium falciparum parasite through the examination of blood from children in West Africa. "Our results shed new light on a mechanism for the weakening of adaptive immunity by invasive parasites," said Aïssatou Diawara, co-author of the study, in a statement.
Increased Mortality Among Children

Passed through the bites of vectors—infected female Anopheles mosquitoes—five parasitic protozoa from the Plasmodium cause malaria in human beings. Two of these, Plasmodium falciparum and Plasmodium vivax, pose the biggest threats.
According to the WHO, P. falciparum caused for 99.7 percent of infections in Africa, 71 percent in the Eastern Mediterranean, 65 percent in the Western Pacific, and 50 percent of cases in South-East Asia. P. vivax was responsible for 70 percent of the malarial infections in the Americas. Also, children formed nearly 67 percent of all Malaria deaths worldwide in 2018.
Evading the Immune System
For the study, the team analyzed blood samples of children from rural Burkina Faso, West Africa. They investigated the immune responses and genomes of the children before, during, and after the infection. It was discovered that the parasite targeted a group of genes known as microRNAs, which are small molecules that play a key role in the moderation of genes associated with immune responses.
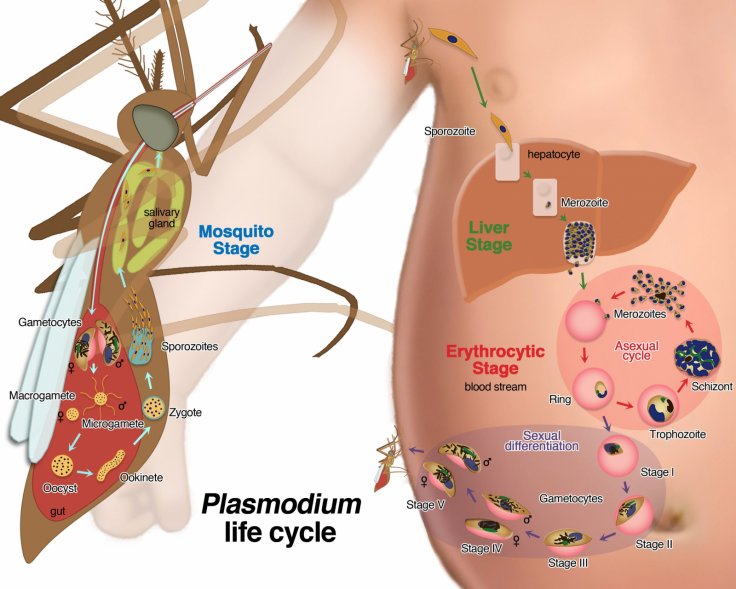
The action on the genes leads to cell death of the cells of the adaptive immune system when they were present alongside the Plasmodium parasite. After the parasite evades the cellular response of the blood, it can swiftly multiply and attack other blood cells.
An interesting observation made was that few of the microRNAs are still under the genetic control of the body. This, according to the authors, can illustrate why the coping ability towards the diseases among individuals and populations varies. "This could explain why it takes years for children to develop immunity and why vaccines do not provide long-term protection," said Mame Massar Dieng, co-author of the study.
Unequal Burden On African Countries
Of all regions in the world, the burden Sub-Saharan Africa is the largest, with negative socio-economic consequences making outcomes worst. Efforts for the development of sustainable and therapeutic strategies against malaria have been impeded by the limited knowledge of the sources that lead to the variations in immune responses to the infection.
Outlining the succeeding plans for the research, Youssef Idaghdour, co-author of the study, explained, "The next step for the team will be to perform more functional tests and to gain a better understanding of why certain groups of people in Africa are more immune to the disease than others."
Idaghdour also pointed out that the ongoing novel coronavirus pandemic has stalled the efforts towards the eradication of malaria."Due to the impact of COVID-19 on healthcare systems, and screening and prevention programs, the burden of malaria could be worse in the coming years and it is our hope that this research can contribute to reaching the long-term goal of malaria elimination," he concluded.









