In the fight against COVID-19, scientists and doctors are implementing new methods, therapeutics and drugs to treat patients. While some have had success, many haven't lived up to expectations. But convalescent plasma treatment has been touted as "game-changing" with the U.S. Food and Drug Administration (FDA) issuing an emergency use authorization (EUA) for hospitalized COVID-19 patients, on Sunday, August 23.
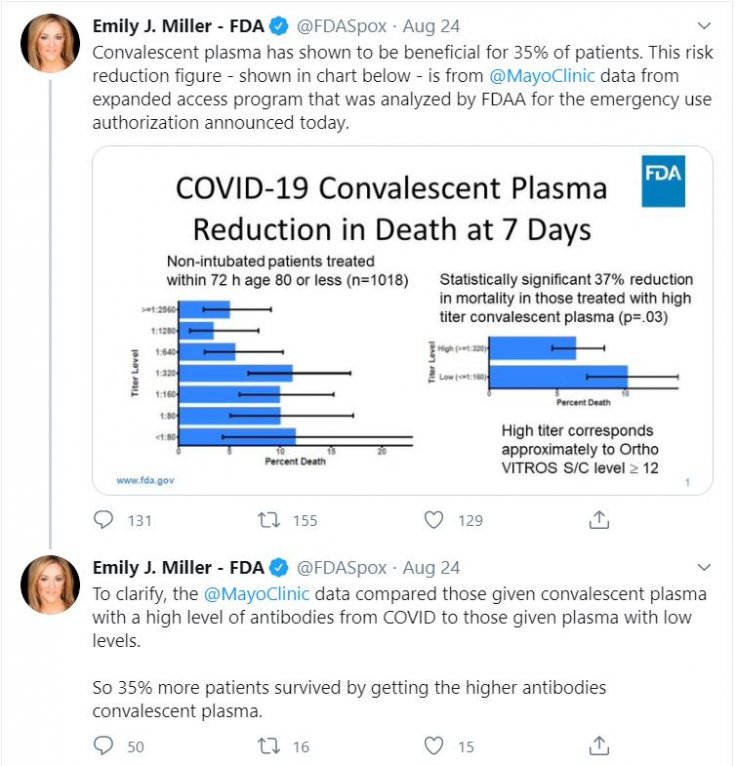
During the announcement of EUA, FDA Commissioner Dr Stephen M Hahn said 35 percent of COVID-19 patients would have been saved if they are administered plasma while U.S. President Donald Trump called it a "tremendous" number. Health and Human Service Secretary Alex Azar also lauded the number.

However, following their remarks, Hahn had to face backlash from health experts and scientists, forcing him to reverse his stance on Monday night.
What Is Convalescent Plasma Therapy?
When a patient recovers from a disease, the person's body produces antibodies, a protein to fight off the infection and create immunity. The blood from the patient is called convalescent plasma as it contains the antibodies required to fight the infection or in this case COVID-19. Plasma is the liquid portion of the blood that contains the antibodies.
In many countries, the experimental therapy is already being used including in India, where authorities have seen a significant reduction in mortality rate. In the U.S., Mayo Clinic too has conducted the study.
Although Mayo Clinic provided the plasma therapy to over 70,000 patients, in their study, researchers didn't have a controlled trial, where a group of patients would have been given the plasma therapy while the other group would be treated with placebo.
Instead, a small group of COVID-19 patients under 80 years old, who did not require ventilation at that time, were studied. There were other variables that were not considered as well that could have an impact on the study — such as how sick the patients were when they were given plasma therapy.
As per a New York Times report, the Mayo Clinic study never mentioned the 35 percent number that Hahn or Trump administration used while announcing the EUA. While the number is actually significant, it hasn't been fully confirmed by the scientists yet. The FDA authorization letter, memo by FDA scientists and not even the research paper had the number. And that puzzled scientists. They believe FDA "grossly misrepresented" the data.
"For the first time ever, I feel like official people in communications and people at the F.D.A. grossly misrepresented data about a therapy," Dr Walid Gellad of Center for Pharmaceutical Policy and Prescribing at the University of Pittsburgh said.
Where Did It Come From?
If the 35 percent improvement was not mentioned in any of those official documents then where did it come from? An FDA spokesperson said the number was derived from a graph that was mentioned in the application for emergency authorization.
However, the graph did not contain such figures. It said patients who received a low level of antibodies through plasma therapy had 63 percent survival probability while high-level antibodies increased the rate up to 76 percent.
Dr Peter Marks, FDA Director of Center for biologics, evaluation and research, on Monday (August 24) said that the agency conducted its own analysis of data from published studies and the Mayo Clinic's research. "There appears to be roughly a 35 percent relative improvement in the survival rates of patients who received the plasma with higher versus lower levels of antibodies," he said in a statement, adding that size of the benefit although varied.
Backlash and Reverse in Stance
The interpretation didn't go down well among the health experts. Johns Hopkins' professor, Dr Arturo Casadevall, who was one of the main authors of the Mayo Clinic study, said he was surprised by the interpretation. "That was not the way that I would have worded it," he said.
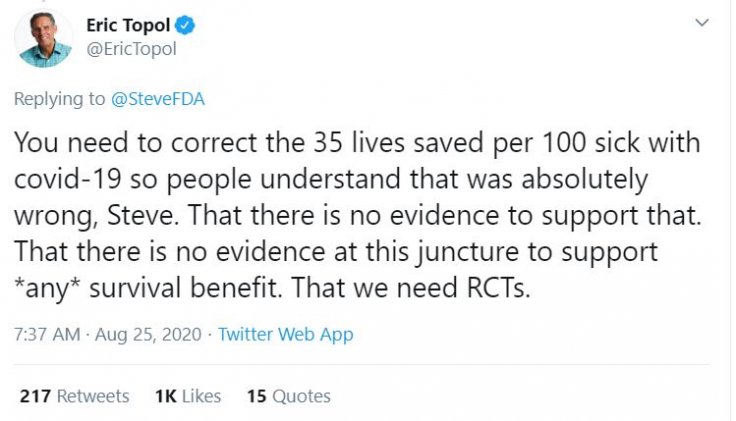
He added that the data showed a reduction in mortality rate when high-level antibodies were given and that was significant. But Casadevall said that "until we have a randomized controlled trial, we don't know definitively."
Dr Erik Topol, Scripps Research Director & Founder, termed it as an "egregious public statement". Hahn issued a clarification in a tweet later, saying the "criticism was entirely justified".









