The World Health Organization (WHO) said vaccines—from essential childhood immunization to seasonal flu—have prevented at least 10 million deaths between 2010 and 2015. Now, as the world is trying to win the battle against novel Coronavirus, 167 vaccine candidate are in development to prevent COVID-19.
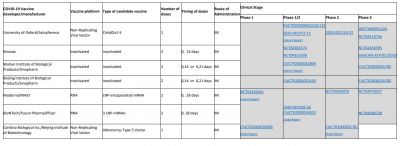
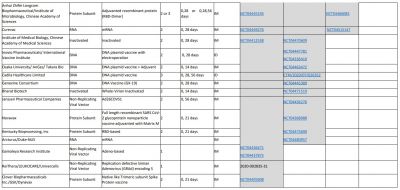
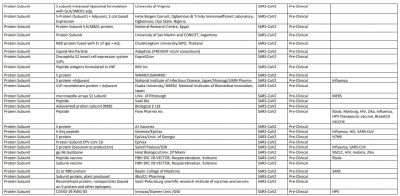
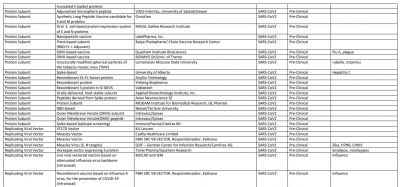
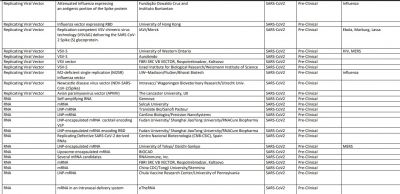
According to the WHO, 30 vaccine candidates are in clinical evaluation, while some of them are considered the most promising against SARS-CoV-2. That list includes Oxford's AZD1222/Covishield, Sinovac Biotech vaccine, Moderna vaccine, Ad5-nCoV by CanSino Biologics, Sinopharm vaccine, Pfizer vaccine, and Johnson & Johnson's vaccine Ad26.
Vaccine development during a pandemic is always challenging. The issues also include the production capacity for the entire global population that is 7.5 billion approximately. So now, the questions are—when will a vaccine be ready how will it reach the world? And how many vaccines should be produced to cover everyone in the world?

Coronavirus Vaccine
Around 80 nations have submitted expressions of interest to protect their people through joining the COVAX Facility. The mechanism is designed to guarantee rapid, fair, and equitable access to Coronavirus vaccines for every country in the world, rich and poor—to make swift progress towards slowing down the pandemic. These countries would finance the vaccines from their own public finance budgets.
These countries have partnered with up to 92 low and middle-income nations that could be supported through voluntary donations to Gavi's COVAX Advance Market Commitment (AMC). Together these alliances will represent over 60 percent of the world's population. Dr. Seth Berkley, CEO of Gavi, the Vaccine Alliance said, "COVAX is the only truly global solution to the COVID-19 pandemic."
A new landmark collaboration between the Bill & Melinda Gates Foundation, the Serum Institute of India (SII), Gavi, and the Vaccine Alliance will accelerate manufacturing and delivery of up to 100 million doses of AstraZeneca or Novavax's candidate vaccines, if successful, for low- and middle-income countries in 2021.
Dr. Berkley said the new collaboration will help "ensure we have the additional manufacturing capacity to begin producing doses for every country, not just the wealthy few." Separate agreements between Gavi, CEPI, and AstraZeneca guarantee further 300 million doses of their candidate vaccine, if successful, for the COVAX Facility.
Promising Vaccines and Production Efforts
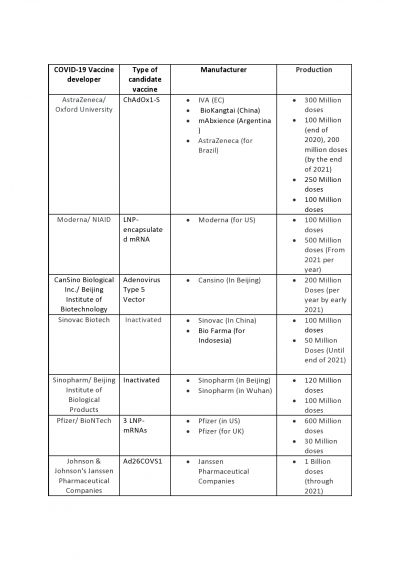


- University of Oxford/AstraZeneca: The vaccine called ChAdOx1-S (AZD1222) is being developed by the British university in collaboration with pharmaceutical company AstraZeneca. AstraZeneca has concluded an agreement with the European Commission (EC) to supply at least 300 million doses of its COVID-19 vaccine. It will allow the EU member states to redirect doses to other countries in Europe. The British-Swedish biotech company has entered into an agreement with Chinese vaccine manufacturer BioKangtai to produce 100 million doses of the vaccine in China by the end of 2020.
- As per this deal, the company will deliver the first 100 million doses this year and 200 million doses by the end of 2021. As reported, AstraZeneca will work with Mexico and Argentina to boost the vaccine supply to Latin American countries. Both South American countries are expected to produce between 150 million and 250 million doses of the adenovirus-based vaccine at no profit starting in the first half of 2021.
- Brazilian President Jair Bolsonaro earmarked 1.9 billion reais, or about $350 million, to buy and later produce the AstraZeneca vaccine in his country. Reuters reported that Brazil's agreement calls for an initial 100 million doses that will be enough to vaccinate about half of the country and when the supply runs out, it will then produce the vaccine on a local level.

Moderna YouTube Grab
- Moderna/National Institute of Allergy and Infectious Diseases: The mRNA-1273 vaccine, developed by the U.S. biotech company Moderna and NIAID was the first to be tested on humans in the U.S. In August, the U.S. government awarded the company an additional $1.5 billion in exchange for 100 million doses of the vaccine. Moderna CEO Stéphane Bancel earlier said that "In terms of the capacity, I'll confirm what we have said before, which is for 2021, the 500 million doses per year continue to be our base plan." In its website, the company wrote, "Manufacturing scale up with Lonza on track to supply 500 million to 1 billion 100 μg doses per year."

- CanSino Biological Inc./Beijing Institute of Biotechnology: This candidate vaccine is developed using weakened adenovirus. As reported earlier, Qiu Dongxu, executive director and co-founder of CanSino said that its new factory under construction in China will allow the company to produce 100 to 200 million doses of the Coronavirus vaccines per year by early 2021. However, CanSino has won a patent approval from Beijing for its COVID-19 vaccine candidate Ad5-nCOV that was submitted for application on March 18 and was approved on August 11.

Sinovac Biotech Sinovac Website
- Sinovac Biotech: The potential coronavirus vaccine is being developed by Beijing-based Sinovac Biotech. The biotech company has received $15 million in funding to advance the development of its CoronaVac. As reported, the company is making a commercial vaccine production plant to manufacture up to 100 million doses of CoronaVac per year. Recently, Sinovac has also committed to providing 50 million doses of CoronaVac to Indonesia's government from November to March. Indonesia's state-owned Bio Farma, which would get priority access until end-2021, said that 50 million bulk would come in stages: 10 million for each month starting in November.
Sinopharm Sinopharm website
- Sinopharm: This vaccine candidate is an inactivated form of SARS-CoV-2 that is developed by the state-owned China National Pharmaceutical Group (Sinopharm). Liu Jingzhen, Chairman of Sinopharm Group recently said the price of the vaccines will not be high after they go into the market, with two shots costing less than 1,000 yuan ($144). A report claimed that while a workshop in Beijing able to produce 120 million doses per year has passed a biological safety inspection from the authorities and getting ready for production, Liu another workshop of Sinopharm in Wuhan is able to produce 100 million doses per year.

Pfizer YouTube Grab
- Pfizer/ BioNTech /Fosun Pharma: Pfizer and German biotechnology company BioNTech are developing a vaccine that uses messenger RNA. Pfizer, the American pharmaceutical company said it could roll out up to 20 million doses of the Coronavirus vaccine, BNT162, for emergency use by the end of 2020.Earlier, the company said they have come to an agreement with the U.S. government for up to 600 million doses of the vaccine. Pfizer and BioNTech also announced an agreement with the U.K. to supply 30 million doses of their mRNA-based vaccine candidate.
-

Johnson & Johnson Wikimedia Commons
- Johnson & Johnson: Ad26.COV2-S, this vaccine is also being developed from a weakened adenovirus by Janssen Pharmaceutical Companies. J&J's Chief Scientific Officer Paul Stoffels said, the company is scaling up capacities in other countries "to further our goal to supply more than one billion doses of the vaccine globally through the course of 2021, provided the vaccine is safe and effective."
Russia Coronavirus Vaccine YouTube Grab/Sputnik
- Russia Sputnik V: Even though many researchers are doubtful about this Russian vaccine, and disagree to consider Sputnik V as a potential COVID-19 vaccine (due to lack of evidence), Russia said it is expecting to produce between 1.5 million and two million doses per month of its vaccine by the year-end. Russia's industry minister Denis Manturov said that the country is aiming to gradually increase the production up to six million doses a month.
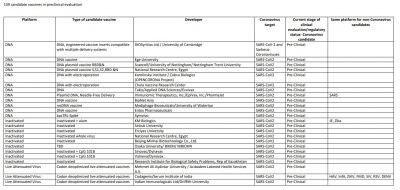
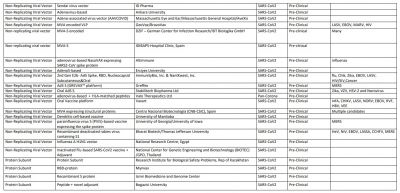
All these vaccine developers are manufacturing millions and billions of doses of the vaccine but will it be enough to cater nearly 7.5 billion population? We just have to wait and hope for the best. However, here is the list of other COVID-19 vaccines: