As the Coronavirus cases are rising every day in the world, especially in the US, which is struggling to curb the virus spread, an experimental hormone therapy showed signs of hope to speed the recovery time for the COVID-19 patients.
Dr. Tim Rich from Duluth's Essentia Health and Dr. David Ingbar from the University of Minnesota had studied for years whether a common thyroid hormone could be used as a treatment for acute respiratory distress syndrome (ARDS). They received federal approval in 2019 to test the therapy. But when the Coronavirus pandemic hit the world it caused a sudden surge in ARDS—a medical condition in which fluid collects in the air sacs of the lungs, depriving organs of oxygen.
Then the researchers started their trial program and Duluth patients were among the first to get an experimental treatment that uses thyroid hormone to mitigate lung failure.
A Magic Treatment?
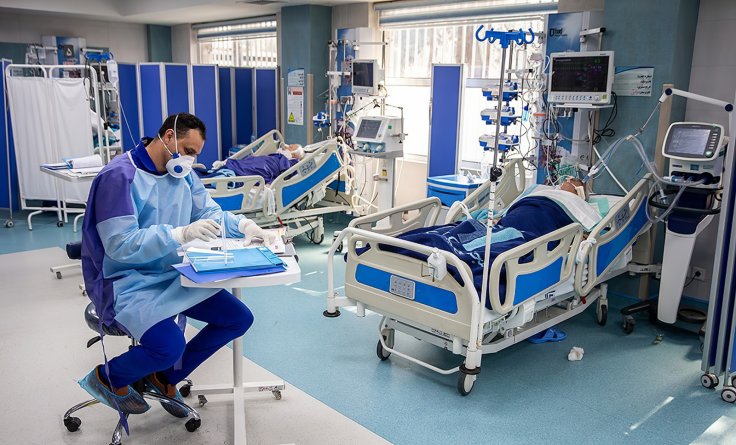
Bob Schlicht, a Coronavirus patient, was in a hospital intensive care unit on a ventilator as he was facing breathing troubles. While his wife, Kristine Smoley, prepared to hear the worst news from the hospital, a nurse from Essentia Health called her and told her about the experimental treatment that could save her husband.
"They asked if I wanted to consider signing off on an experimental treatment for him. A treatment that had never been done before" said Kristine Smoley. It was her husband who was the first patient to receive the treatment. Even though an experimental treatment is "scary," according to the wife of the Coronavirus patient, "I don't know that I really had an option. Because the other option wasn't good."
One of the researchers behind the experimental treatment Rich, who is a pulmonologist said that "This is not a designer drug. This is something we know the lung needs and uses."
As of now in the US, over 9 million cases were reported from all the states and the COVID-19 killed more than 231,000 Americans till Monday, November 2. But neither the US nor other nations have successfully developed a vaccine against the virus. Only antiviral drug remdesivir has received FDA approval for its use in hospitalized Coronavirus patients. Treatments like plasma infusion are still considered experimental and only used under emergency authorization.
However, Rich and Ingbar discovered that during the H1N1 pandemic in 2009, the victims' lungs lacked a thyroid hormone called T3. As reported, Ingbar said, "A part of this acute lung injury with ARDS is the lungs get leaky, and they tend to fill with fluid. That makes it really hard to get oxygen in or carbon dioxide out."
The 68-year-old Bob Schlicht, who was Itasca County's first known positive case of COVID-19, was coughing when he was first going to an emergency in March. A US woman Mary Ellen Evangelista found that her brother Tim White, 51, who was a corrections officer at the Moose Lake prison, was tested positive for the infection in April. Similar to Bob Schlicht, Evangelista's brother's condition was also very critical.
White spent more than a month in the Duluth hospital and took the experimental treatment. But after he returned home, the chest x-ray showed that he has comparatively healthy lungs. Citing his case, Ingbar said, "This is really a much faster recovery than we see with typical ARDS." After receiving the treatment, Bob Schlicht also said that he feels 100 percent healthy.
T3 Worked
The timing of the recovery of those who received the experimental hormone treatment suggested that T3 actually worked, but doctors cannot rule out completely the fact that the men recovered due to other medical care too.
However, the trial was paused following the treatment of Schlicht and White for a safety review. Now as the researchers got a go-ahead signal, their next step will be to recruit 68 patients with ARDS from COVID-19 or other causes for the FDA-approved study and then compare 50 patients who receive supplemental T3 with 18 who receive standard care.









