Regulators in the UK have issued a warning stating that people with a history of "significant" allergic reactions should avoid taking the Pfizer-BioNTech COVID-19 vaccine after two NHS workers who received the shot reported "adverse reactions".
According to NHS England, two staff members who received the vaccine developed by Pfizer and its German partner BioNTech suffered an allergic reaction. However, recent reports revealed that both the healthcare workers, who had a history of significant allergic reactions, are now recovering well.
Do Not Take the Vaccine Now
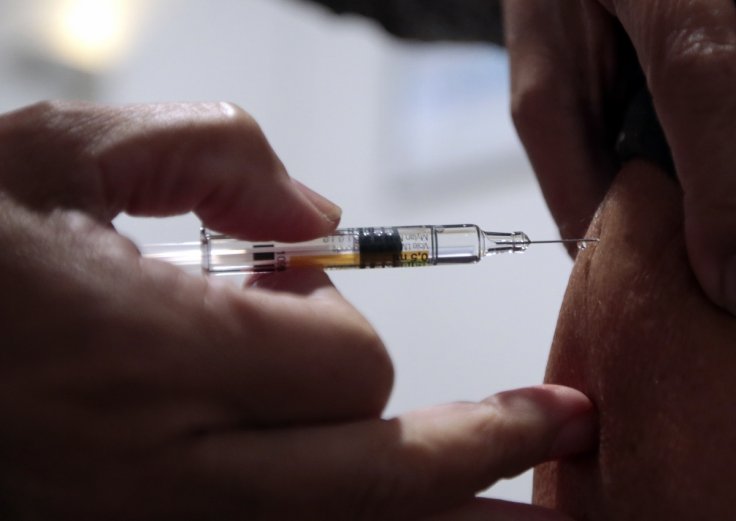
After the unexpected incident, the NHS was told by the Medicines and Healthcare products Regulatory Agency (MHRA) that anyone with a history of "significant" allergic reactions should not receive the Pfizer vaccine. The NHS said that all trusts involved in this vaccination program in the UK had been informed.
According to Professor Stephen Powis, national medical director for the NHS, the MHRA has advised on a "precautionary basis" that people who earlier witnessed an allergic reaction to medicines, food, or vaccines should not get this vaccine after two people with a history of significant allergic reactions "responded adversely yesterday [Tuesday]".
After the UK became the first country to approve a vaccine against the COVID-19, the European regulators advised caution. At that time, European Medicines Agency (EMA) also said in a statement that its longer approval process was more appropriate as it was based on more evidence and needed more checks than the emergency procedure chosen by the UK authority.
Peter Liese, an EU legislator, earlier said that he considered the approval of the vaccine by MHRA to be problematic. "I recommend that EU member states do not repeat the process in the same way. A few weeks of a thorough examination by the EMA is better than a hasty emergency marketing authorization of a vaccine," he added.
Vaccination in the UK
People in the UK were vaccinated at 70 hospitals across the nation on Tuesday. Among all those people from the high-risk groups who received the jab, 90-year-old Margaret Keenan said she felt "privileged" after becoming the first person in the world to get the Pfizer-BioNTech vaccine shot against the Coronavirus caused disease outside the clinical trials.
As of Wednesday, the UK reported more than 1,754,000 COVID-19 cases and over 62,000 deaths. Health Secretary Matt Hancock has cautioned that the guidance still needs to be followed as the vaccine is distributed over the next few months. He told the MPs in the Commons that people should "temper our joy and enthusiasm" at the beginning of the rollout with the need to keep each other safe from the disease. "Let's not blow it since we can see the answer is on the horizon", said Hancock.
However, many healthcare experts have advised people to follow all the safety measures, including wearing masks and maintaining social distancing, even after taking a vaccine. Patrick Vallance, who is UK Government's chief scientific adviser, said that the country is unlikely to get back to a semblance of normality before spring and people might still need face masks in winter 2021.









