Pitting one pathogen against another to neutralize or destroy one of them is not unheard of. Studies have also demonstrated that the antibodies meant to counter a particular virus may have an inhibiting effect on another, including the SARS-CoV-2 coronavirus. Now, a new study has shown promising results that suggest that the antibodies developed against the SARS-CoV—a relative of the SARS-CoV-2 that causes Severe Acute Respiratory Syndrome (SARS)—could help fight COVID-19.
According to the study by a team of Chinese researchers, the antibodies extracted from serum samples acquired from patients who had been infected by SARS-CoV during the 2003 pandemic, were found to effectively neutralize the SARS-CoV-2 in cultured cells. The scientists also found that mice and rabbits immunized with a viral protein particle, receptor-binding domain (RBD), from a strain of SARS-CoV they showed strong antibody responses.
Determining Cross-reactivity of Antibodies
For the study, the authors examined 20 convalescent serum samples procured from patients who had been infected with the SARS 17 years ago. Next, they tried to ascertain the cross-reactivity for antigen proteins derived from four sections of the coronavirus spike protein—a structure that the SARS-CoV-2 uses to infect cells. The section included S ectodomain (S), S1 subunit, RBD, and S2 subunit.
Though all samples of serum reacted strongly with the proteins S and S2, the reaction with the S1 and RBD proteins were weaker. Another test employing a using a technique known as a single-cycle infection assay was performed.
The test helped determine that the convalescent SARS-CoV sera as able to efficiently prevent the pseudoviruses—viruses that cannot manufacture viral surface proteins on their own—of both SARS-CoV and SARS-CoV-2 from infecting cells. However, the inhibition of the activity of the SARS-CoV-2 was effectively lesser.
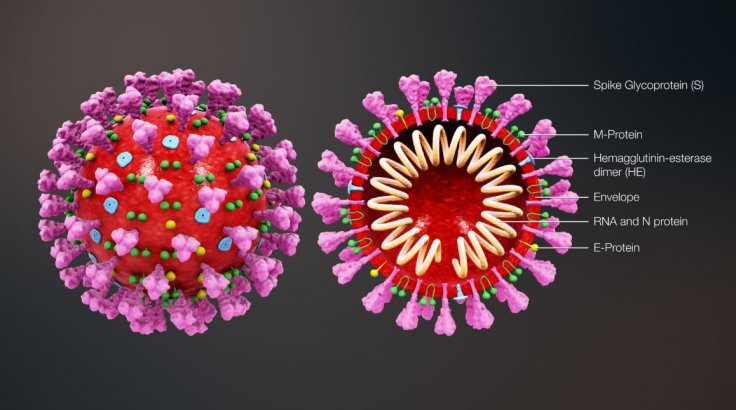
"All patient sera reacted strongly with the S1 subunit and receptor-binding domain (RBD) of SARS-CoV, cross-reacted with the S ectodomain, S1, RBD, and S2 proteins of SARS-CoV-2, and neutralized both SARS-CoV and SARS-CoV-2 S protein-driven infections," wrote the authors.
Strong Immune Response in Mice and Rabbits
The researchers immunized mice and rabbits with RBD collected from a strain of SARS-CoV that is known to infect the Himalayan palm civet to verify their findings in animals by studying cross-reactivity. As the RBD is the least protected protein site on the spike of both the viruses.

It was discovered that the anti-RBD serum produced from SARS-CoV showed strong cross-reaction with SARS-CoV-2. This suggests that a vital antigen constituent is genetically preserved within the RBD sites of both the viruses. Another important finding was that the mice and rabbit immunized with civet RBD showed stronger antibody responses against the SARS-CoV-2 than those immunized with RBD from a human strain of the SARS-causing virus.
The authors highlighted that antibody-dependent enhancement (ADE)—binding of a virus to particular antibodies that aids in its entry into host cells—was not seen during the study. "Although antibody-dependent infection enhancement (ADE) was not observed during our studies with the human and animal serum antibodies, this effect should be carefully addressed in vaccine development," stressed the authors.









