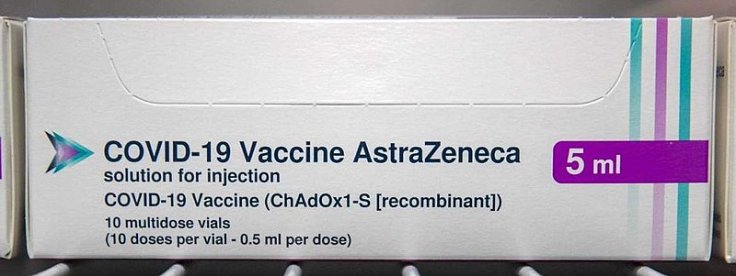
Health officials in South Africa said that they are halting the use of the Oxford-AstraZeneca COVID-19 vaccine. This decision was taken after a study showed that the jab can provide minimal protection against mild and moderate cases of the new Coronavirus variant which was first identified in the African country.
South Africa Minister of Health Dr. Zweli Mkhize said on Sunday, February 7, that the hold would be temporary until scientists figure out how to solve the issue. However, the minister also assured that the country will move forward with the deployment of vaccines made by Pfizer-BioNTech and Johnson & Johnson.
"What does that mean for our vaccination program which we said will start in February? The answer is it will proceed. From next week for the next four weeks we expect that there will be J&J vaccines, there will be Pfizer vaccines," said Mkhize.
South Africa plans to vaccinate at least 67 percent of the country's population -- around 40 million people -- by the end of 2021. The African country has recorded almost 1.5 million novel Coronavirus infections and over 46,000 COVID-19 deaths since the beginning of the pandemic.
AstraZeneca Vaccine and South African Variant
According to the new data, which was released on Sunday, two doses of the COVID-19 vaccine developed by the University of Oxford and pharmaceutical company AstraZeneca can provide only "minimal protection" against the mild and moderate diseases from the South African variant, dubbed B1351 -- first found in mid-November 2020.
The study suggesting the less efficacy of the vaccine has not been published. It included almost 2,000 volunteers who were an average of 31 years old. About half of these participants received the Oxford-AstraZeneca vaccine, while others received a placebo.
Researchers said in a news release that the viral neutralization against the new South African variant was "substantially reduced" when compared to the earlier strain of Coronavirus. However, the efficacy of the vaccine against the severe COVID-19 cases, hospitalization and death were not assessed.
The findings of the study by researchers from South Africa's University of Witwatersrand and others, as well as from the University of Oxford have been submitted for peer-review. According to Oxford, a preprint will be released soon.
However, AstraZeneca stated that the company is now working closely with the health ministry of South Africa on how to support the evaluation against severe COVID-19 caused by the new variant. The company believes that the vaccine will still protect against severe disease from the South African variant, specifically when the dosing interval is eight to 12 weeks.
As the new findings raised concern among researchers, Maria Van Kerkhove, World Health Organization's technical lead for Covid-19 said on Sunday that an independent WHO's vaccine panel will meet Monday, February 8, to discuss the AstraZeneca vaccine. The panel will also talk about the findings of the study and what it means for vaccines going forward.
Kerkhove said: "Some preliminary studies suggesting reduced efficacy. But again, those studies aren't fully published yet." She explained that it would be critical to have more than one safe and effective vaccine and the world cannot rely on only one product.









