As the world longs for Coronavirus vaccine and a hundred percent effective treatment, Singapore's Tychan, a biotechnology company, will begin human trials in 23 volunteers for possible monoclonal antibody treatment.
The first phase of the six-week trial will begin next week, infectious diseases expert Ooi Eng Eong and the co-founder of Tychan, told media on June 10. The trial, which will be conducted by SingHealth's investigational medicine unit, will determine the effectiveness of the immune system protein TY027 that targets the SARS-CoV-2, the virus which causes COVID-19.
"One obvious thing is that a lot of the Covid-19 patients get sick for a very long time, and some of them even get very severe respiratory disease, so much so that you need oxygen ventilators to help them tie through this critical period, without which they would die," Eong said in a statement.
"You could use it to treat all COVID-19 patients and prevent them from getting severe disease. You could also give it to those who are going to get severe disease and prevent them then from sliding further in their respiratory function," he added.
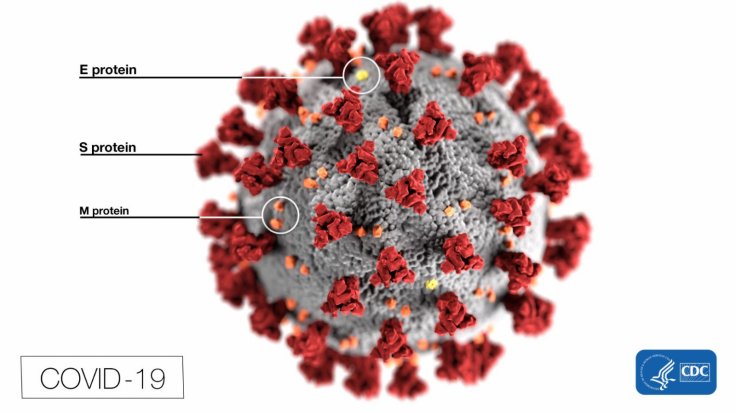
Antibody Treatment
A body produces antibodies to fight infection. Scientists and researchers believe that antibody-based treatments promise better results in infectious diseases. Similarly, with COVID-19, such treatment should yield a positive result. Hence, if the trial is successful, the drug can be used for protection against the COVID-19 infection.
Through the trial, monoclonal antibodies will be isolated and manufactured to treat Coronavirus patients. At present, hydroxychloroquine, an anti-malarial, remdesivir, a drug used in Ebola patients, and a cocktail of different drugs are used to treat patients with variable results.
"Rapidly developing a cure for COVID-19 is exactly the raison d'etre of Tychan. "Whilst still a few months away from knowing if we are successful, we are hopeful because of our experience in Zika and Yellow Fever," Teo Ming Kian, chairman of Tychan, said in a statement.
He added that the company reached the human trial phase in just four months when it generally takes about 12-18 months.
Temporary Vaccine
If the trial is successful, the drug can be used as a temporary vaccine as antibody drugs remain effective in the body for about two-three weeks per dose.
"If the drug is indeed safe enough, we could, for instance, give it to people who travel to places with a lot of COVID-19 cases. This could be used to prevent infections when they are away from Singapore," Eong said, adding that healthcare workers will be the first to get it to prevent the infection, if it works.
Currently, there are more than 300 ongoing trials worldwide for a possible Coronavirus treatment and vaccine.
There has been a proliferation of more than 300 clinical trials worldwide for the coronavirus. Another Singapore team, Agency for Science, Technology and Research (A*Star), is also in the development of similar antibody treatment.









