The World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) have come up with conflicting guidelines on COVID-19 vaccines for pregnant women. While the CDC approves it, the WHO is cautious and is not recommending it if the person is not a healthcare worker. Hence, for potential recipients, it is more confusing than ever.
The debate, for now, is over Moderna and Pfizer-BioNTech's mRNA-based vaccines. Both the vaccines have been rolled out across the world and are endorsed by the WHO. Both are over 90 percent effective in stopping the COVID-19 disease. But when it comes to endorsing it for pregnant women, the WHO has advised against it while most of the country's health ministries have recommended it.

The problem here is not the efficacy but the potential side-effects that could harm the fetus and the mother. While both the companies have said that it would not have any adverse reaction and any impact on the fetus, the WHO is not counting it out due to the lack of data.
Study on Pregnant Women
There have been studies showing pregnant women at higher risk of contracting novel Coronavirus than other women in the same age group. According to the Harvard Medical School, pregnant women are at higher risk for hospitalization, intensive care and breathing support. They are also at higher risk of death. Considering the factors, they should be on the priority list, right? This is where it gets murkier.

Pregnant women were not part of the controlled trial groups for both Moderna and Pfizer. Hence, it cannot be surely said that there would be no effects on the women or the unborn babies. That is the primary reason why the WHO is advising against it.
"Pregnant women are at higher risk of severe COVID-19 than non-pregnant women, and COVID-19 has been associated with an increased risk of pre-term birth. However due to insufficient data, WHO does not recommend the vaccination of pregnant women at this time," the WHO said in its recommendation for both Pfizer and Moderna's vaccines.
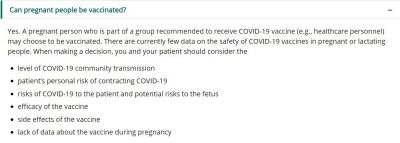


However, the WHO isn't the only one advising against it. Countries like the UK and India have also recommended pregnant women not to take the vaccine if they are not part of a vulnerable group until more data is available. Besides Pfizer and Moderna, the UK regulatory body has approved the AstraZeneca-Oxford University vaccine that is based on an Adenovirus-based vector. India has also approved two non-mRNA vaccines.
mRNA Based Vaccines
While pregnant women do receive vaccines for flu and tetanus, diphtheria, pertussis (TDP), the two COVID-19 shots will be mRNA based and are rather new. The vaccines did not have any adverse side-effects nor impacted fertility during the animal trial. As for human trials, some women became pregnant and 18 of them received the vaccine. However, data on them is awaited.

However, scientists believe mRNA-based vaccines should not be a cause for concern as they do not have any live virus particle that could reach the placenta and infect the baby. Furthermore, the mRNA particles that are used in the vaccine are eliminated by the body within days or even hours. The advantage is that the immunity generated by the mother's body can cross the placenta and keep the baby safe. Hence, the CDC, the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists (ACOG) recommend that pregnant women receive the COVID-19 vaccination.
"There are no documented risks to the fetus, there are no theoretical risks, there's no risk in animal studies from the vaccines. The more that I think about it, the more disappointed and sad I feel about it," Dr Anne Lyerly, a bioethicist at the University of North Carolina, Chapel Hill, told the New York Times.
The bottom line, for now, is to consult with obstetricians before deciding on vaccination.









