In a major breakthrough, scientists at the Utrecht University, Erasmus Medical Center, and Harbour BioMed (HBM) have identified an antibody that blocks and prevents SARS-CoV-2 (COVID-19) virus from infecting living cells.
The antibody - 47D11, targets the COVID 19 causing virus's 'spike protein', which it uses to invade cells and insert its genetic material.
The scientists, who conducted the tests on mice cells found that the antibody 47D11 attaches itself to COVID-19 spike protein and prevents it from invading living cells – thereby effectively neutralizing it.
The discovery, published online in Nature Communications, could prove to be major breakthrough towards developing a fully human antibody to treat or prevent the respiratory disease COVID-19 caused by the novel coronavirus SARS-CoV-2 that till now has killed over 249,061 people world-over.
In the study, researchers observed the antibody, if injected into humans is capable of altering the 'course of infection' or protect an uninfected person exposed to someone with the virus.
In the paper titled, "A human monoclonal antibody blocking SARS-VoV-2 Infection," the European scientists revealed that the discovery provides a strong foundation for additional research to characterize the antibody (47D11) and begin development as a potential COVID-19 treatment.
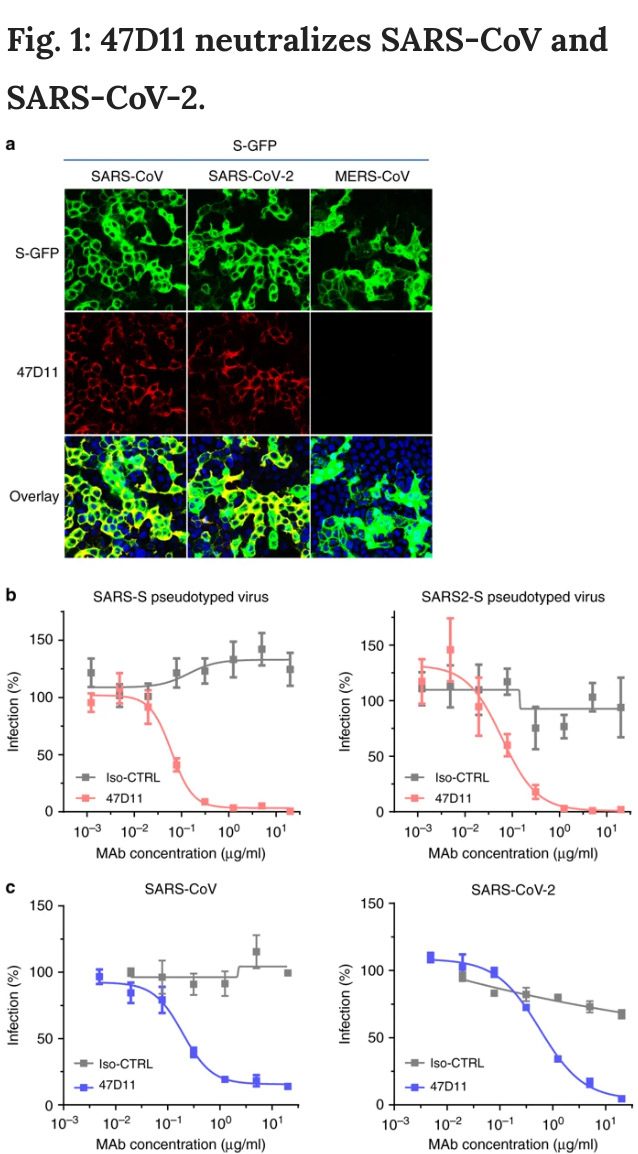
As part of the joint study, the researchers used a collection of SARS-CoV (that emerged in 2002/200) antibodies and from it identified an antibody that also neutralizes infection of SARS-CoV-2 in cultured cells.
During the tests, the researchers found that the antibody binds to a domain that is conserved in both SARS-CoV and SARS-CoV-2, explaining its ability to neutralize both viruses. "This cross-neutralizing feature of the antibody is very interesting and suggests it may have potential in mitigation of diseases caused by future-emerging related coronaviruses," said Berend-Jan Bosch, Associate Professor, Research leader at Utrecht University.
The researchers generated the COVID 19 virus blocking antibody using Harbour BioMed's H2L2 transgenic mouse technology.
"The antibody used in this work is 'fully human,' allowing development to proceed more rapidly and reducing the potential for immune-related side effects," said Frank Grosveld, PhD. co-lead author on the study, Academy Professor of Cell Biology, Erasmus Medical Center, Rotterdam and Founding Chief Scientific Officer at Harbour BioMed.
Conventional therapeutic antibodies are first developed in other species and then must undergo additional work to 'humanize' them.









