As scientists continue to unravel the mysteries of the SARS-CoV-2 virus, one important element of its highly efficient design has left the scientific community startled -- its spike protein. The appendage-like structure is an important weapon in its pathogenic arsenal as it enables the COVID-19-causing coronavirus to gain entry into cells. Now, an international team of researchers has found that glycans—sugar molecules attached to proteins—play an integral role in the spike's ability to invade cells.
According to the study published in the journal, ACS Central Science, focusing on glycans can provide potential targets for therapies and vaccines to aim at. "This work presents hitherto unseen functional and structural insights into the SARS-CoV-2 S protein and its glycan coat, providing a strategy to control the conformational plasticity of the RBD (receptor binding domain) that could be harnessed for vaccine development," wrote the authors.
How the Virus Invades Cells
Spikes are protein structures that cover the surface of the SARS-CoV-2 virus. These structures enable the virus in infecting human cells, especially lung cells. ACE2 (Angiotensin-converting enzyme 2), an enzyme that is found on the cell membranes of cells in the lungs, intestines, arteries, kidneys and heart, is targeted by the spike.
The infection of cells begins when the spike protein tethers to the ACE2 cell receptors. As the infection progresses, it aids in the catalyzing process that boosts the release of the coronavirus' genetic material into the cell. However, more information about the binding process between the spike and the ACE2 receptors remains unknown and it is this area that researchers have directed all their efforts at.
Recent studies have discovered that spikes can adapt itself into 10 different structural states and also posses "pockets" that can be exploited to inactivate the virus. Keeping in line with such findings, the current study has uncovered that sugar molecules known as glycans that are found on the spikes play an important role in the spike's ability to overpower cells.
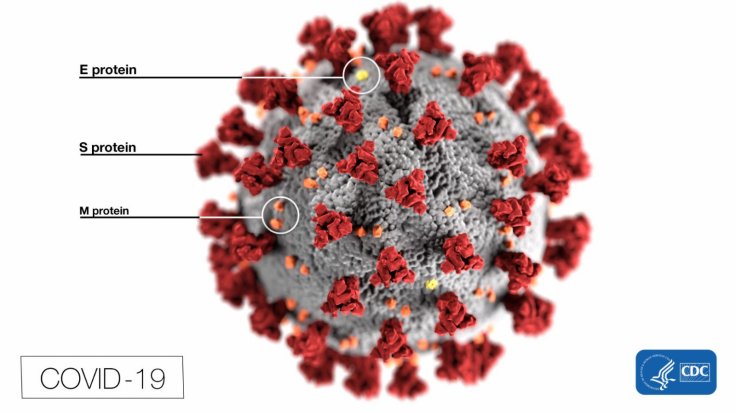
Not So Sweet Sugars
Glycans are sugar compounds covering the surface of proteins. Much like all viral proteins, the SARS-CoV-2 spike protein has a thick layer of glycans on its surface. Attached to specific regions of the spike, they protect it from the immune system of the host. The authors tried to ascertain whether certain glycans decorating the SARS-CoV-2spike protein play an active role in the infection process.
For the study, the team used glycomic and structural data to construct molecular dynamics simulations—computer simulation model for the analysis of biomolecules—of the novel coronavirus' spike protein rooted in its viral membrane. These models provided a detailed representation of every atom in the spike protein.
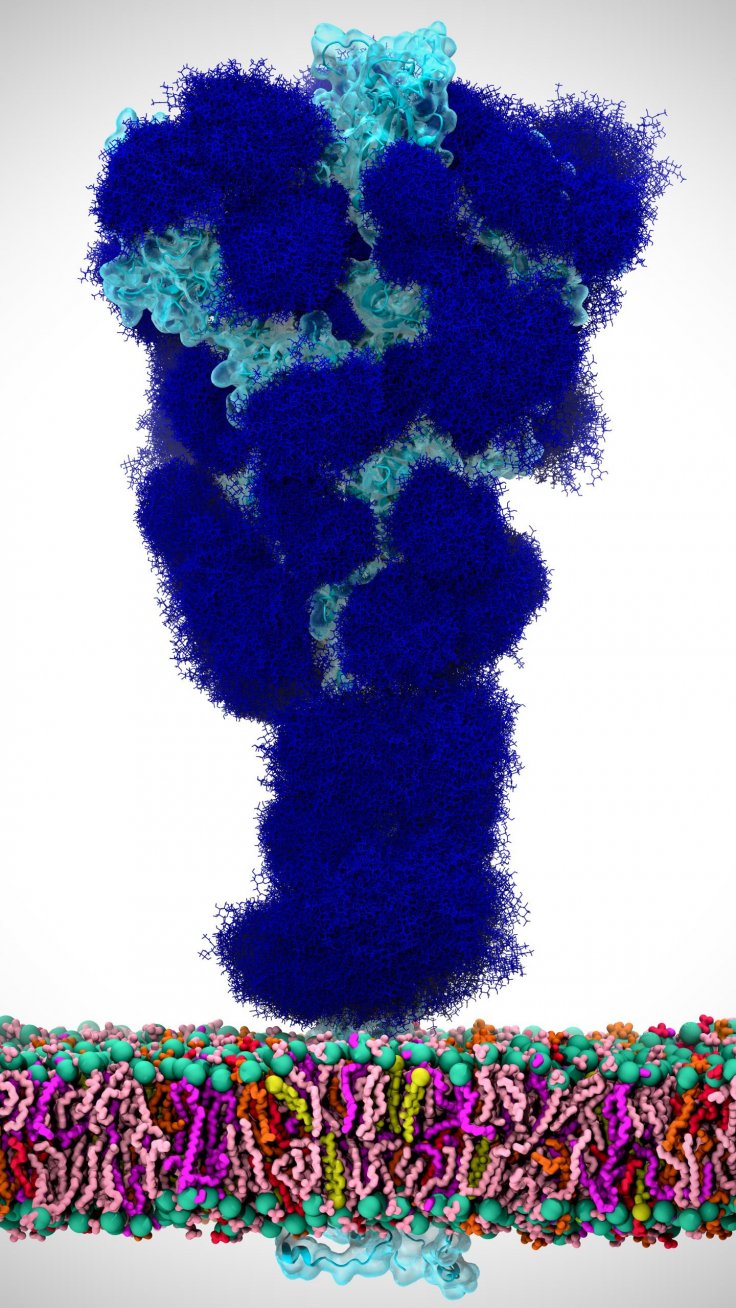
It was discovered that glycans known as 'N-glycans' that are connected to the spike protein at two specific regions—N165 and N234—stabilized the change in the shape of the spike when its RBD was exposed, thereby, assisting in the infection process. RBD is the part on the spike that helps it latch on to receptors. The authors wrote: "We reveal an essential structural role of N-glycans at sites N165 and N234 in modulating the conformational dynamics of the spike's receptor-binding domain (RBD), which is responsible for ACE2 recognition."
Identifying Vulnerabilities
During the simulations, the authors also identified sites on the spike that were not covered by glycans and may be vulnerable to antibodies. Through laboratory experiments utilizing biolayer interferometry—an optical analytical technique to measure interactions between biomolecules—the made another interesting finding. They learnt that manipulating/mutating the spike protein in a manner that glycans at N165 and N234 were no longer present, reduced binding to ACE2 significantly.
The scientists hope that the results of the study establish the basis for the development of new strategies to combat the pandemic. "Additionally, end-to-end accessibility analyses outline a complete overview of the vulnerabilities of the glycan shield of the SARS-CoV-2 S protein, which may be exploited in the therapeutic efforts targeting this molecular machine," they wrote.









