The first results of the most awaited human trials of Moderna's coronavirus vaccine candidate have induced antibody response in a bunch of healthy volunteers after an early study, after which shares rose steeply.
On Monday, the Massachusetts-based biotech explains that this was a small study of a trial primarily focused on safety. However, these positive results do not confirm if this vaccine candidate prevents people getting infected by the novel coronavirus.
Trials were conducted by the US National Institutes of Health (NIH). The company plans to start a mid-stage study soon after which a late-stage trial would be done by July. Moderna aims to get ready for emergency use of the vaccine this fall, announced the company.
The early trial had enrolled 45 healthy volunteers, with age ranging from 18 to 55, while randomly giving one of the three dosages for two shots that is, 25 micrograms, 100 micrograms and 250 micrograms. This vaccine was "generally safe and well tolerated," said Moderna.
Stocks
David Rosenberg, the chief economist of Rosenberg Research, told The Washington Post in an email that investors got rewarded a "triple whammy of good news."
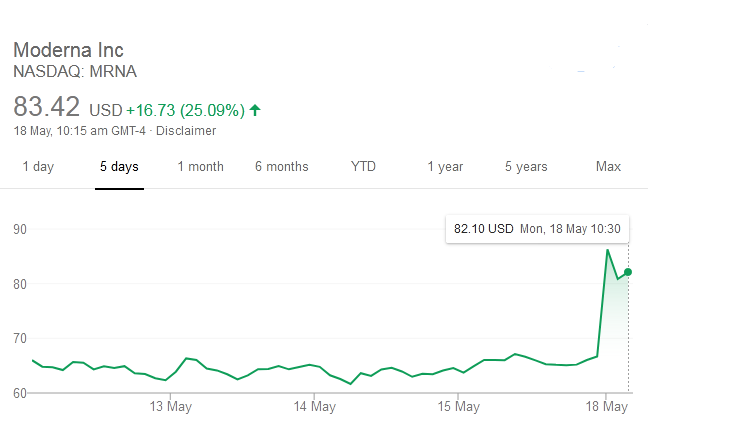
He further said that, there there was a relief as majority of the US states were reopening their economies, which includes 75 percent of California. There was also "growing hope" that the vaccine getting close, sooner than later, while he said that Fed Chairman Jay Powell had told investors that central bank's checkbook remains wide open, that hinted at more monetary policy stimulus.
All of them developed antibodies
All these participants developed detectable antibodies, while the company said that it saw a dose dependent increase in "immunogenicity."
The weakest dose strength given to 15 volunteers also produced antibodies in similar levels as those who already recovered from COVID-19, after they received both the doses.
The company tweeted a summary of the data:
Side Effects
Among those who had highest dosage level (250 micrograms), three persons had severe side effects that are not life-threatening. However the company did not describe these side effects.
All volunteers showed neutralizing antibodies in almost equal or above levels usually seen in "convalescent sera," according to Moderna, while they had data on the same for eight people.
NIH helped in testing of the vaccine in mice. They were infected with SARS-CoV-2 (novel coronavirus) after giving them the vaccine candidate. It "prevented viral replication in the lungs" of mice, the company said, adding that the reported early human data on neutralizing antibody levels had consistency with data from mice.









