American cancer specialists have unveiled a new 'Trojan horse treatment' that doubles the survival rate of patients suffering with triple-negative breast cancer.
The drug identified as Trodelvy is also proven to work fine with HR-positive breast cancer treatments that comprise nearly 70 % of all cases. The latest trial of the drug was conducted on the stage IV patients, most of whom had stopped responded to other traditional treatments.
With the aim to directly supply chemo to the tumor, the Trodelvy uses artificial antibodies, which are quite similar to the ones naturally produced by the immune system. These antibodies then carry a chemotherapy medicine and attach themselves to the cancer cells.
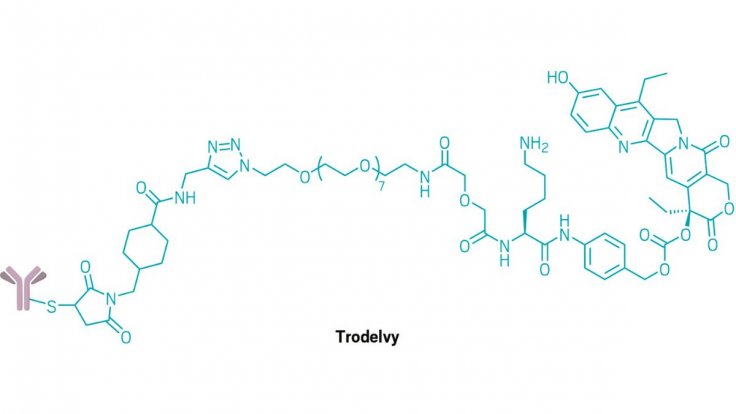
After observing several cases, the researchers deduced that the drug also known as sacituzumab govitecan-hziy can successfully fight cancer for at least a year. Dr Jane Mowe Meisel, an American cancer specialist praising the study at the American Society of Clinical Oncology in Chicago, believes that Trodelvy will be able to offer the advanced breast cancer patients "a one-in-five" chance of the disease not progressing in a year.
She further added that this is a huge achievement in cancer research as the treatment will provide hope to patients who have already completed their chemotherapy and have 'no options left,' the Daily Mail reported.
Commenting on the study, Professor Nick Turner, a breast cancer expert at the Institute for Cancer Research in London, welcomed the move as he mentioned that while the drug is not a cure but it certainly "buys more time" for the patients.
Negotiations for the authorization of the drug in UK are scheduled to begin on Tuesday with the National Institute for Health and Care Excellence (NICE). This spending body of the National Health Services of UK had majorly disappointed the cancer research communities in UK when it previously rejected the drug deeming it "too expensive" to be a "cost-effective use of NHS resources".
Jo Taylor, founder of After Breast Cancer Diagnosis and patient advocacy group MET UP UK, that mainly represents metastatic breast cancer patients, was delighted by the new treatment but disagreed with NICE's decision to refuse the authorization of the Trodelvy.
"I have friends who are desperate to access this drug, and considering how quickly the disease progresses, even a two-week delay is too much. These people need it immediately," he said.

"By making this decision, NICE is taking away a vital lifeline that could offer TNBC patients further months or years of life," said MET UP UK, "We protest loudly against this cruel decision and demand that NICE reverses it and approves Trodelvy for use on the NHS."
Some charities like Breast Cancer Now also started a petition in an effort to reverse the decision. For such groups this negotiation is a huge deal.
Gilead Sciences, a US biopharmaceutical giant, is approached for a £40,000-a-year per patient price tag by NICE. Trodelvy was approved in the US by Food and Drug Administration on 13 April last year.








