Among many medicines that have been suggested for COVID-19, none has been as controversial as the hydroxychloroquine (HCQ). The anti-malarial drug was initially claimed to be a miracle cure for the disease. But as per new data, over 100 people who were suffering from COVID-19 have died in the U.S. even after taking the drug or related medicine.
As per the analysis of data from the adverse events reporting system of the U.S. Food and Drug Administration (FDA), it was revealed that 293 people had died in the first half 2020 that involved HCQ. The number of deaths related to the drug was up from 75 compared to the last year's data of the same time, Milwaukee Journal Sentinel revealed. It also found that of the total deaths, more than half had COVID-19.
A total of 6,588 cases of adverse effects were reported in the first half of this year, up from 3,251 of 2019 first six months. The drug had although been used for the treatment of malaria, auto-immune disease and arthritis, a study claimed it to be effective in COVID-19 treatment.
HCQ for COVID-19 Treatment
HCQ was touted as a possible drug for COVID-19 treatment after a study was published in The Lancet journal. But the original study was retracted following a backlash in the medical community. The World Health Organization (WHO) too halted the trials, then resumed again in June and then stopped in July.
"These interim trial results show that hydroxychloroquine and lopinavir/ritonavir produce little or no reduction in the mortality of hospitalized COVID-19 patients when compared to the standard of care. For each of the drugs, the interim results do not provide solid evidence of increased mortality," WHO said in a statement.
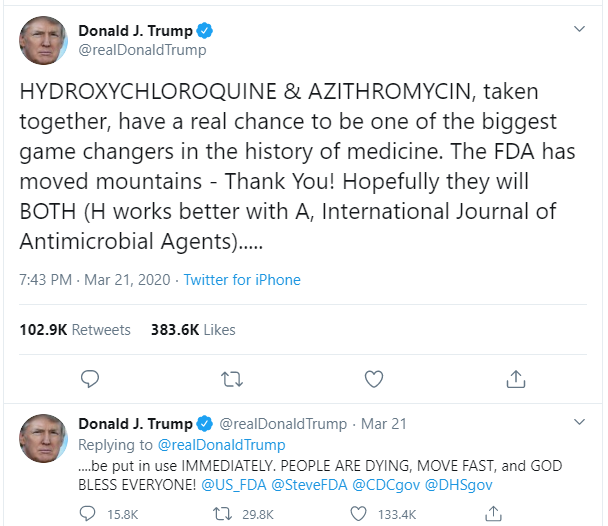
Despite WHO and the U.S. Centre for Disease Control and Prevention (CDC) saying otherwise, U.S. President Donald Trump contradicted the evidence on multiple occasions and said he was taking the drug as a preventive measure. The FDA had issued an emergency use authorization for the use of HCQ in COVID-19 but later revoked after many patients reported arrhythmia (irregular heartbeats).
Dr Michael Carome, Director of the patient advocacy group Public Citizen and a former FDA adviser told the Milwaukee Journal Sentinel that the rise in adverse effects was to be blamed on Trump's "reckless promotion" of the drug. Many other medical professionals including Infectious-Disease expert Dr Anthony Fauci and his White House Coronavirus Task Force team member Dr Deborah Birx have pointed out that evidence doesn't support that HCQ is effective in treating COVID-19.
Continued Debate
However, despite the evidence and expert opinions against it, HCQ has continued to create a divide not only in the U.S. but also in other countries. A team of researchers from the University of Oxford, Wellcome Trust and the Mahidol Oxford Tropical Medicine Research Unit (MORU) has urged not to rule out the use of HCQ in COVID-19.
The team is conducting a trial known as COPCOV in Thailand and is aiming to enroll over 40,000 health care workers with close contact with COVID-19 patients to understand the efficiency of the drug in treating the disease.
"By the time patients are admitted to hospital virus multiplication is well past its peak and inflammation in the lungs and other complications may prove lethal. At this stage the steroid dexamethasone, which reduces inflammation, saves lives but the antivirals hydroxychloroquine and chloroquine do not," explained Dr Nick Day, one of the researchers.
Hence, there is still a grey area that has been used by HCQ advocates like Trump to contradict scientific evidence. Dr Nicholas White, Principal Investigator of the study, told Newsweek that politicization of the drug has been the major issue in conducting clinical trials.
"There is also some indication for a small benefit in the post-exposure prophylaxis studies reported to date, but nothing that can be regarded as reliable or definitive," he said advocating randomized trials.








