US biotech firm Moderna has sent its coronavirus vaccine to US researchers after a six-week work on it, which means the next stage of vaccine trials could begin sometime in April though the final nod for its use may be some time next year.
In a statement, Moderna (MRNA) said the first lot of its wuhan coronavirus vaccine, called mRNA-1273, has been sent to the National Institute of Allergy and Infectious Diseases (NIAID) for its approval to go ahead with human clinical trials.
In the next phase, the Boston-based biotech company has to produce a vaccine with mRNA (or messenger ribonucleic acid), a molecule vital to the proper functioning of the body's cells to make vaccine that instructs cells in the body to make proteins to fight the disease.
First phase of trials
Moderna said the first step of trials of the vaccine would involve testing on 25 healthy humans to see if it produces an immune response that protects against the virus. NIAID Director Anthony Fauci said the clinical trials could start by the end of April, known as the "first step" in making a vaccine available.
The company claimed that the vaccine was developed within 42 days of the company obtaining genetic information on the coronavirus, whereas similar stage was achieved in the case of SARS after almost 20 months. However, it's not the first company to have claimed a fast-paced breakthrough.
Indian company's claims
Earlier, India-based drugs manufacturer, Serum Institute of India (SII), announced last week that it has developed a vaccine in collaboration with American biotechnology company, Codagenix, which may be ready by early 2022. The company's CEO Adar Poonawalla said that China could be a potential site for clinical trials and has already been tested in mice and primates.
"While several efforts have been made to finding a cure as well as in controlling the outbreak, this is the first vaccine-virus strain to progress to the pre-clinical trial phase," the company said, as quoted by PTI.
Trial phase
After the successful clinical trial, the vaccine undergoes further testing for a wider application on humans and then goes for federal approval before making the vaccine is made available in the market, even when the trail phase is carried out on emergency basis.
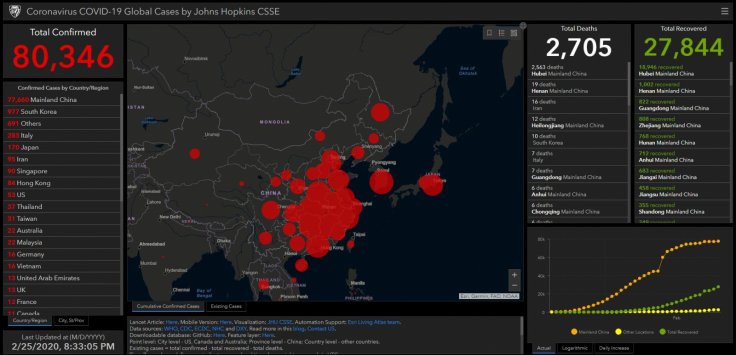
The Wuhan Coronavirus or COVID-19 has already claimed the lives of more than 2,500 victims, with 80,000 cases registered all over the world. In view of its fast-paced spread, many pharmaceutical companies around the world are working overnight to develop a vaccine.








